is nacl a homogeneous mixture
So the different individual ions are completely intermixed and surrounded by water molecules on the molecular level. For every one sodium atom there is one chlorine atom that cant change.

Solved 2 Which Of The Following Is A Homogeneous Mixture Chegg Com
Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase.
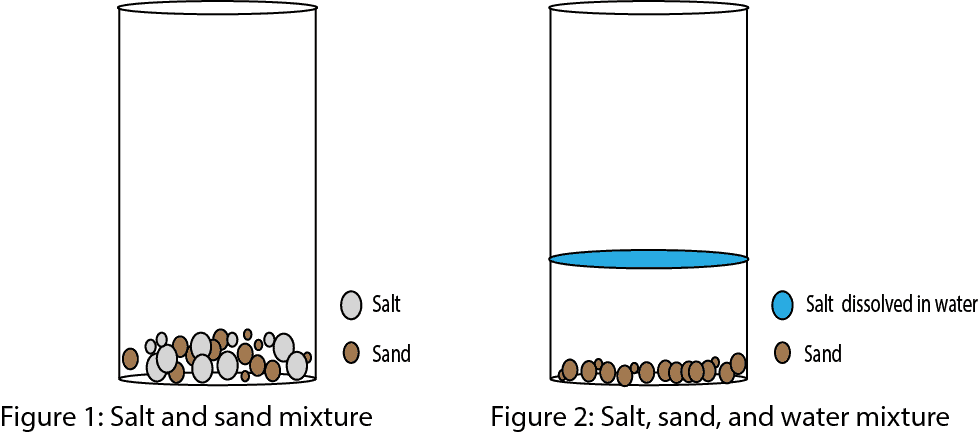
. Homogeneous mixture could be exemplified as a sugar solution or salt solution whereas Mixture of salt and sand could be used as an example of Heterogeneous mixture. Sodium chloride is called ordinary table salt. Solutions and Mixtures Scientists say that solutions are homogenous systems.
Water is a pure material as well. Sodium chloride is a chemical compound not a mixture. Saltwater is a basic mixture of salt NaCl and water H2O.
As NaCl completely dissolves in water aqueous sodium chloride is a homogeneous mixture thus giving a single visible step. It is because the paint is a kind of suspension. Assuming you mean table salt NaCl no.
An example of a homogeneous mixture is H2ONaCl or NaCl aq. Further Explanation Mixture A mixture is a substance that is made up of two or more substances that are not chemically combined and in fixed proportions. So if the salt in the shaker contains NaCl KI and anti-caking agent and appears uniform throughout the.
Salt water is made by mixing salt NaCl in water. Compound-has a formula associated with it. It is a homogeneous mixture and a heterogeneous one.
Is NaCl solution a homogeneous mixture. Compound- has a formula 2 or more capitol letters associated with it. These mostly contain water but not always.
Why is NaCl a mixture. This qualifies as a mixture since evaporation allows reformation of sodium chloride solid again thus separating the components NaCl and H_2O via physical means. The particles here are greater than 5 x 10-7 m in size which means that you can see the particles with the naked eye.
It is a homogeneous mixture and a heterogeneous one. Homogeneous mixtures can be further classified into. Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase.
NaCl is a solid at room temperature with a very high melting point 801 C similar to the melting points of silver 96178 C and gold. A homogeneous mixture is a solution with multiple compounds. Sodium Chloride is not a homogenous or heterogeneous mixture it is not a mixture at all.
Since it has a uniform and definite structure it is called a substance. The question arises whether the saltwater is homogeneous or heterogeneous. Is table salt a homogeneous mixture.
All samples of sodium chloride are chemically identical. Is a solution always heterogeneous. The Na and Cl- ions are completely dissociated from one another in solution and form electrostatic interactions with hydrogen and oxygen atoms in the water.
Expert Answers info A homogeneous mixture is composed of a single visible phase while a heterogeneous mixture has two or more visible phases. Recall that a solution is a homogeneous mixture composed of two or more substances. NaClaq is a homogeneous mixture.
A difference between the two is that concentration is same everywhere in case of a homogeneous mixture whereas in case of a heterogeneous mixture the concentration varies from place to place within a mixture. An aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase. Why do painters always mix the paint before using it.
Both sodium chloride samples are chemically similar. Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase. Salt water is made by mixing salt NaCl in water.
It is known as a homogeneous mixture. Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase. The Na and Cl- ions are completely dissociated from one another in solution and form electrostatic interactions with hydrogen and oxygen atoms in the water.
So this depends on the concentration of the mixture. It is considered a substance because it has a uniform and definite composition. A mixture in which its constituents are not distributed uniformly is called heterogeneous mixture such as sand in water.
In a homogeneous mixture the concentration is the same at every place. Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase. It is a compound as it is in definite proportions.
A difference between the two is that concentration is same everywhere in case of a homogeneous mixture whereas in case of a heterogeneous mixture the concentration varies from place to place within a mixture. We know that when a material or substance is in the same state and is uniformly distributed it is a homogeneous mixture. Tap water is also a solution and is uniformly distributed throughout the mixture so we can say that tap water is a homogeneous mixture.
All solutions are homogeneous mixtures. Is air a heterogeneous mixture. Why is tap water homogeneous.
Aqueous sodium chloride is a homogeneous mixture because NaCl completely dissolves in water thus giving a single visible phase. The concentration varies from place to place and elements to elements in the mixture. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture.
Is NaCl a Mixture. If the salt in your question is sodium chloride it is an ionic compound made up of sodium ions and chloride ions. In your case in order for sodium chloride solution to be a mixture it must contain sodium chloride salt and another substance mixed together such that it appears as if it were only composed of one substance.
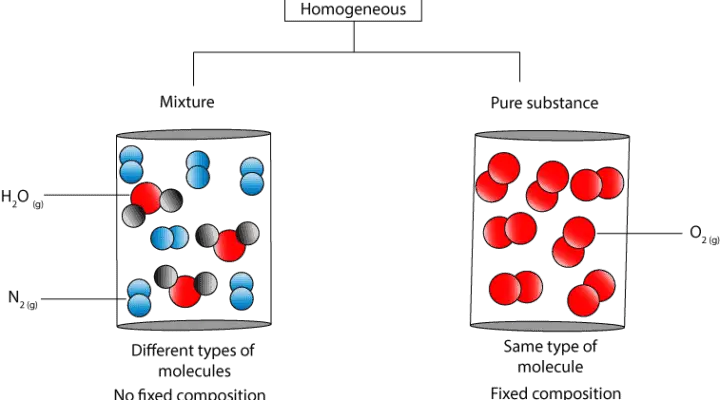
If A Substance Is Homogeneous Is It A Pure Substance

Homogenous And Heterogenous Mixtures Chemistry For Non Majors
Is Sodium Chloride A Mixture Socratic

What S A Mixture How Does Heterogeneous Differ From Homogeneous Mixture And How Can Mixtures Be Separated
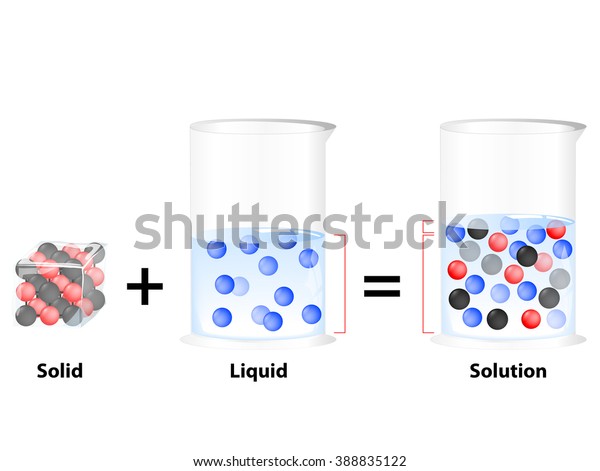
Solution Homogeneous Mixture Substance Dissolved Another Stock Illustration 388835122

3 5 Pure Substances And Mixtures Chemistry Libretexts

Solutions Solutions Homogeneous Mixture Of Two Or More Substances Consist Of A Solute And A Solvent Properties Of A Solution Solutions Have Variable Ppt Video Online Download

What Is A Homogeneous Mixture Definition And Examples Homogeneous Mixture Heterogeneous Mixture Compounds And Mixtures

Consider The Following Substance I Saline Clutch Prep

What Are Mixtures Definition Overview Expii
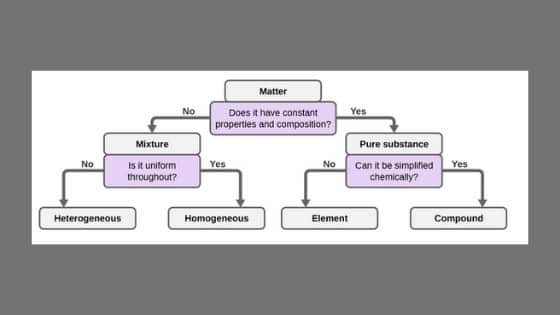
Homogeneous Mixture And Heterogeneous Mixture Ncert Books

Elements Compounds And Mixtures Oh My Ppt Download

Element Compound Heterogeneous Mixture Or Homogeneous Mixture

Question Video Identifying The Best Description Of A Solution From A Set Of Descriptions Nagwa
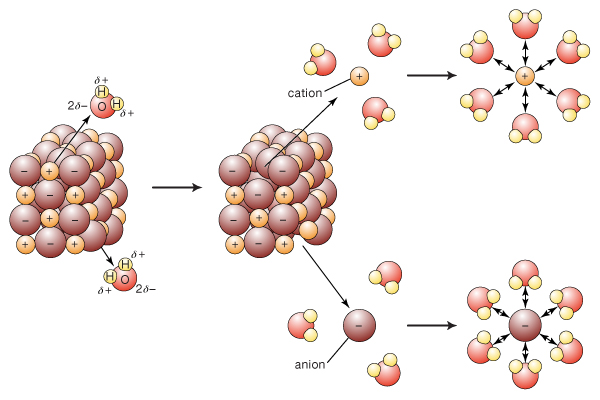
Is Sodium Chloride A Mixture Socratic

2 1 Homogeneous And Heterogeneous Mixtures

Homogeneous Mixture Definition Chemistry Chemistry Dictionary

3 4 Classifying Matter According To Its Composition Chemistry Libretexts

0 Response to "is nacl a homogeneous mixture"
Post a Comment